-
Soybean pod shattering
How easily pods shatter is one of the factors determining seed distribution and yields. In soybean, shattering-resistant lines are much slower in dropping seeds, and generally give more yields than shattering-susceptible lines (Fig. 1).
Our previous studies identified PDH1 as the major QTL controlling the pod shattering. PDH1 encodes a dirigent-like protein, and is thought to regulate lignin biosyntheis and/or cell wall properties. We have been performing further studies on PDH1 to elucidate its functions.

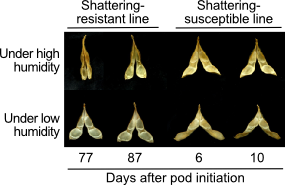
Fig. 1. Shattering-resistant and susceptible lines in soybean.
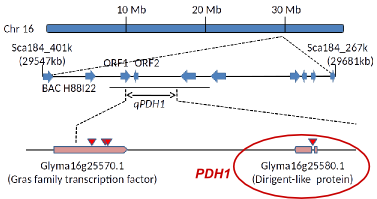
Fig. 2. Fine mapping of PDH1.
References:
Funatsuki H ... and Fujino K. Molecular basis of a shattering resistance boosting global dissemination of soybean. Proc Natl Acad Sci U S A. 2014. 111: 17797-17802. DOI: 10.1073/pnas.1417282111. PMID: 25468966.
Funatsuki H ... and Fujino K. Mapping and use of QTLs controlling pod dehiscence in soybean. Breed Sci. 2012. 61: 554-558. DOI: 10.1270/jsbbs.61.554. PMID: 23136494.
-
Tartary buckwheat bitterness
Tartary buckwheat has a high level of rutin, which is good for human health, and is used instead of common buckwheat to make soba noodles (Fig. 3). The problem was: The process of making the noodles breaks tartary buckwheat cells, and the rutinosidase enzyme leaks. This enzyme converts rutin to quercetin, which is bitter and unpreferable for food.
Recently Manten-Kirari, a tartary buckwheat line with much decreased bitterness, was developed. We have been performing studies on the rutinosidase genes in Manten-Kirari and other tartary buckwheat lines (Fig. 4). We have also been developing a new DNA marker that enables to easily estimate the ratio between Manten-Kirari and other contaminating lines in soba powder.

Fig. 3. Common buckwheat (top-left panel) and tartary buckwheat (bottom-left and right).
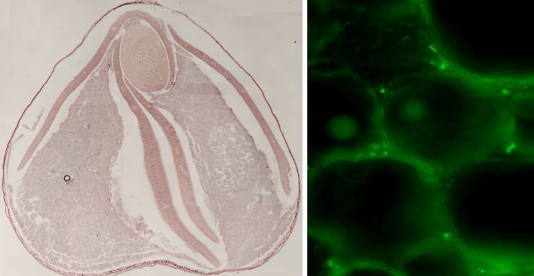
Fig. 4. Expression of the rutinosidase gene in seeds (left panel) and immunofluorescence staining for the rutinosidase in tartary buckwheat (right).
References:
Suzuki T et al. Breeding of 'Manten-Kirari', a non-bitter and trace-rutinosidase variety of Tartary buckwheat (Fagopyrum tataricum Gaertn.). Breed Sci. 2014. 64: 344-350. DOI: 10.1270/jsbbs.64.344. PMID: 25914589.
-
Asparagus sex determination
Garden asparagus is dioecious plant, individuals of which grow either female or male flowers. Female asparagus plants show less stable growth than male, and grow seeds, which raise offsprings and trouble farming. Some male plants can raise only male progenies when crossed, and such parental male plants are called supermale. Commercial asparagus seeds are usually progenies of supermale plants, thus all male.
In asparagus female flowers, stamens stop growing and degenerated, and in male flowers, the pistil stops growing. This results in the morphological differences between female and male flowers (Fig. 5). We have been studying cell death in this process, and effects of phytohormones on this process.
The asparagus sex is known to be determined by the M locus: Plants with the genotype mm are female; Mm male; MM supermale. Recently we have isolated a candidate M locus gene (Fig. 6), and been further characterizing it.
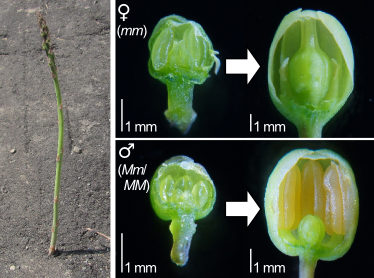
Fig. 5. Asparagus seeding penetrating asphalt (left panel) and asparagus female and male flowers (right).
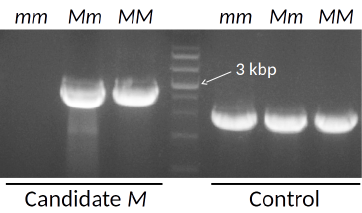
Fig. 6. Detection of the candidate M locus gene with PCR using the genomic DNA of female (mm), male (Mm) or supermale (MM) plants.
References:
Harkess A et al. Sex-biased gene expression in dioecious garden asparagus (Asparagus officinalis). New Phytol. 2015. 207: 883-892. DOI: 10.1111/nph.13389. PMID: 25817071.
Horiuch K, Adachi Y, Kasai N, Yamagishi M, Masuda K. Identification of homozygous male plants by quantitative analysis of a nucleotide sequence linked to the sex-determination locus in Asparagus officinalis L. Journal of the Japanese Society for Horticultural Science. 2011. 80: 308-313. Full text.
Asada Y, Kasai N, Adachi Y, Kanno A, Ito N, Yun PY, Masuda K. A vegetative line of asparagus (Asparagus officinalis) with a homeotic change in flower development is correlated with a functional deficiency in class-B MADS-box genes. Journal of Horticultural Science & Biotechnology. 2006. 81: 874-882. DOI: 10.1080/14620316.2006.11512153.
Jamsari A et al. BAC-derived diagnostic markers for sex determination in asparagus. Theor Appl Genet. 2004. 108: 1140-1146. DOI: 10.1007/s00122-003-1529-0. PMID: 15067401.
-
Plant nuclear matrix constituent proteins
The inner nuclear membrane in animal cells has a fibrous meshwork called nuclear matrix or lamina. Lamina is composed of the lamin protein. No amino acid sequences of plant proteins are very similar to the sequence of lamin, but the plant cell nucleus does have the nuclear matrix. Previously we isolated the plant nuclear matrix constituent protein NMCP. Regardless of the amino acid sequence dissimilarity, higher order structures of lamin and NMCP are predicted to be similar (Fig. 7). The nuclear matrix is necessary for maintaining the nuclear shape and functions. Some mutations in lamin and NMCP cause significant growth defects in individuals in both animals and plants.
We have been visualyzing the NMCP dynamics during mitosis (Fig. 8), and also screenig for NMCP-interacting proteins to gain new insights into the functions of NMCP and other components of the plant nuclear membrane.
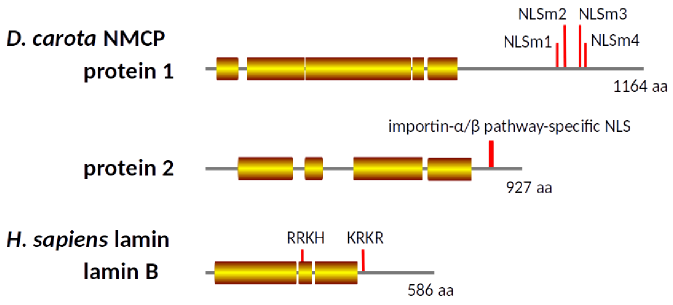
Fig. 7. Predicted secondary structures of NMCP1 (Protein 1), NMCP2 (Protein 2), and lamin B. Predicted coiled-coil regios are indicated as rectangles with color.
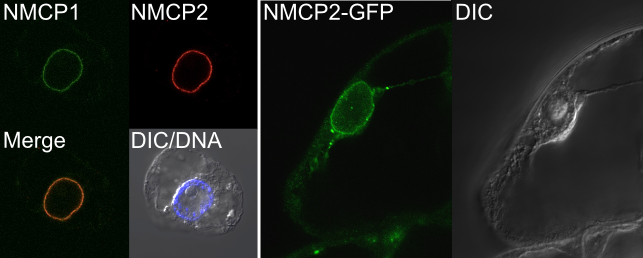
Fig. 8. Immunofluorescence staining for NMCP1 and NMCP2 (left panel) and a subcellular localization study with the NMCP2-GFP fusion protein (right).
References:
Kimura Y, Fujino K, Ogawa K, Masuda K. Localization of Daucus carota NMCP1 to the nuclear periphery: the role of the N-terminal region and an NLS-linked sequence motif, RYNLRR, in the tail domain. Front Plant Sci. 2014. 5: 62. DOI: 10.3389/fpls.2014.00062. PMID: 24616728.
Ciska M, Masuda K, Moreno Díaz de la Espina S. Lamin-like analogues in plants: the characterization of NMCP1 in Allium cepa. J Exp Bot. 2013. 64(6): 1553-1564. DOI: 10.1093/jxb/ert020. PMID: 23378381.
Kimura Y, Kuroda C, Masuda K. Differential nuclear envelope assembly at the end of mitosis in suspension-cultured Apium graveolens cells. Chromosoma. 2010. 119: 195-204. DOI: 10.1007/s00412-009-0248-y. PMID: 19997923.
Masuda K et al. Peripheral framework of carrot cell nucleus contains a novel protein predicted to exhibit a long alpha-helical domain. Exp Cell Res. 1997. 232: 173-181. DOI: 10.1006/excr.1997.3531. PMID: 9141634.
Nomura K, Saito W, Ono K, Moriyama H, Takahashi S, Inoue M, Masuda K. Isolation and characterization of matrix associated region DNA fragments in rice (Oryza sativa L.). Plant Cell Physiol. 1997. 38(9): 1060-1068. PMID: 9360323. Full text.
Masuda K, Takahashi S, Nomura K, Arimoto M, Inoue M. Residual structure and constituent proteins of peripheral framework of the cell nucleus in somatic embryos from Daucus carota L. Planta. 1993. 191: 532-540. DOI: 10.1007/BF00195755.
-
VIP1 in mechanical stress responses
Plants can percieve and respond to mechanical stress such as touch, but it still remains to be seen how plants do so. We have been studying roles of an Arabidopsis thaliana bZIP transcription factor, VIP1, in plant responses to mechanical stress. When Arabidopsis cells are exposed to mechanical stimuli, VIP1 is rapidly imported into the nucleus (Fig. 9). When a VIP1 variant, VIP1-SRDX, which could disturb functions of VIP1, is overexpressed, touch-induced root waving is enhanced (Fig. 10). We have also found that the phytohormone auxin is possibly involved in this phenomenon.
We have been further examining how the subcellular localization of VIP1 is regulated and its relevance to mechanical stress responses. We believe that VIP1 helps us identify novel proteins/genes regulating such responses.
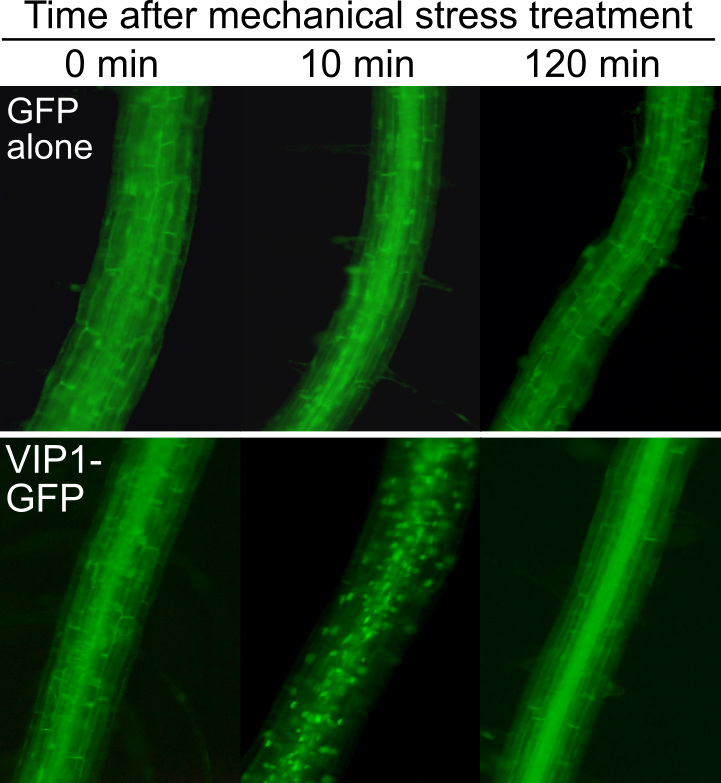
Fig. 9. Transient accumulation of VIP1 in nuclei (dots in the lower middle panel) in root cells exposed to mechanical stress
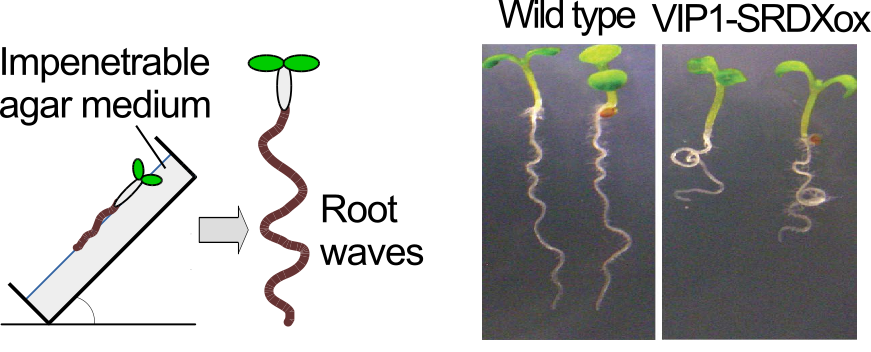
Fig. 10. Touch-induced root waving is more severe in the plants overexpressing VIP1-SRDX (VIP1-SRDXox)
References:
Tsugama D, Liu S, Takano T. VIP1 is very important/interesting protein 1 regulating touch responses of Arabidopsis. Plant Signal Behav. 2016. 11(6): e1187358. DOI: 10.1080/15592324.2016.1187358. PMID: 27171129.
Tsugama D, Liu S, Takano T. The bZIP protein VIP1 is involved in touch responses in Arabidopsis roots. Plant Physiol. 2016. 171(2): 1355-1365. DOI: 10.1104/pp.16.00256. PMID: 27208231.
Tsugama D, Liu S, Takano T. Analysis of functions of VIP1 and its close homologs in osmosensory responses of Arabidopsis thaliana. PLoS One. 2014. 9(8): e103930. DOI: 10.1371/journal.pone.0103930. PMID: 25093810.
Tsugama D, Liu S, Takano T. A bZIP protein, VIP1, is a regulator of osmosensory signaling in Arabidopsis. Plant Physiol. 2012. 159(1): 144-155. DOI: 10.1104/pp.112.197020. PMID: 22452852.