The carbohydrate-related enzymes mainly studied in our laboratory are enzymes that catalyze hydrolytic reactions of carbohydrates (hydrolytic enzymes) and the ones that synthesize polysaccharides (synthetic enzymes that function without sugar-nucleotides), which as a whole encompass a wide variety of enzymes. Enzymes can be categorized based on the chemical reactions they catalyze. This is a classical classification method. Lately, a new method focusing on the “sequence of protein structures (domain or module) involved in the catalytic activity” has been in use. This new method places the basis on the similarity of the amino-acid sequences in protein structures (steric structures are graded higher) and classifies glycoside hydrolase families (GH) by number. At our lab, the main focuses are on carbohydrate-related enzymes belonging to the families of GH13, GH15, GH31, GH66, and GH99. Here are brief introductions of some of these studies.
GH 13
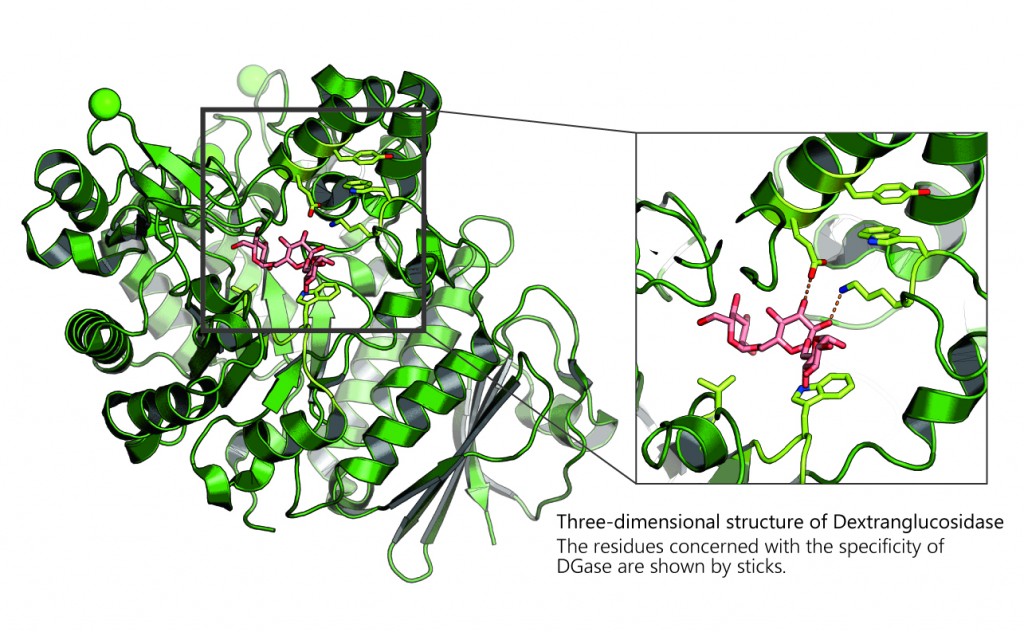
One of the enzymes belonging to GH13 is dextran glucosidase (DGase). DGase is characterized by its inclination to act on long-chain carbohydrates consisting of α-1,6-glucoside linkage, and in the nature, it is an important enzyme involved in the dextran metabolism (dextran is a polysaccharide consisting of α-1,6-glucoside linkage as the main chain). We discovered a structure factor connected with this characteristic feature of DGase. We further figured out its three-dimensinal structure and, based on the information, created a useful enzyme that synthesize oligosaccharides at a high yield rate. Apart from these studies, we have also been studying honeybee α-glucosidase isoenzymes involved in the production of honey.
GH 15
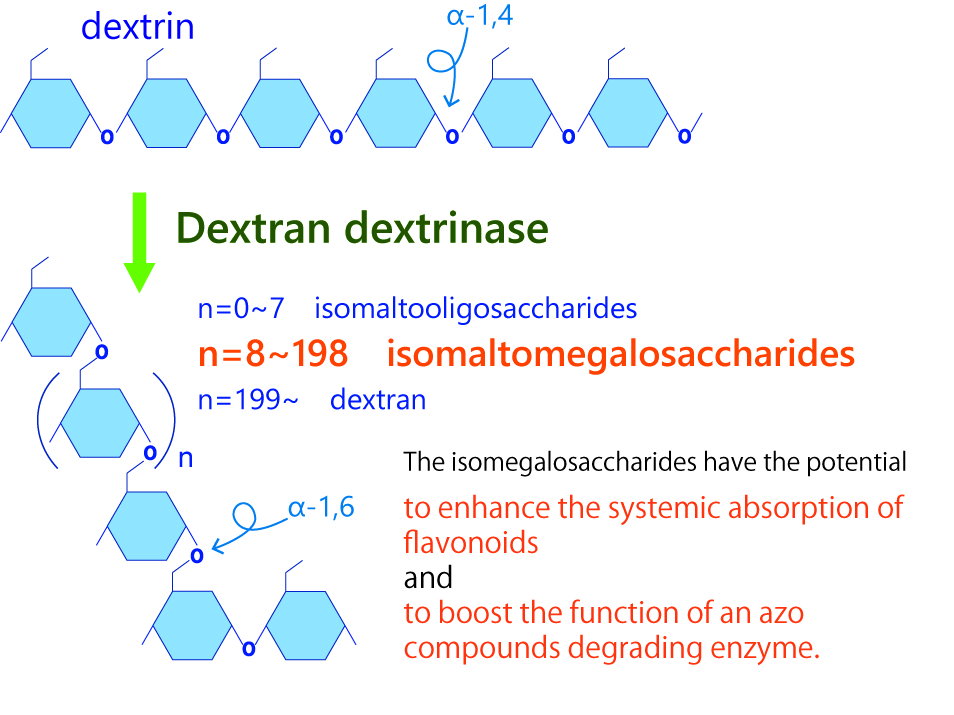
Among GH15, we focus on dextran dextrinase (DDase). DDase is a unique enzyme that produces dextran by acting on dextrin (maltooligosaccharide) comprising of α-1,4-glucoside linkage. Dextran is highly in demand in the academic, medical, pharmaceutical, food, and other areas, and DDase enables the high-yield production of dextran. At our lab, we conducted foundation studies on the production of dextran and, for the first time in the world, succeeded in producing megalosaccharide (a form of carbohydrate composed of 10 – 200 glucoses), which is lower molecular mass than dextran. We discovered new function of megalosaccharide that increases systemic absorption of flavonoid, which is reported for its anti-diabetic and anti-atherogenic functions, and this carbohydrate has put it in the spotlight as an excellent functional food material. It has been further identified with a function to facilitate the enzyme degradation of an environmental pollutant, azo color, raising the expectations for its application to new methods for environmental cleanup.
GH 31
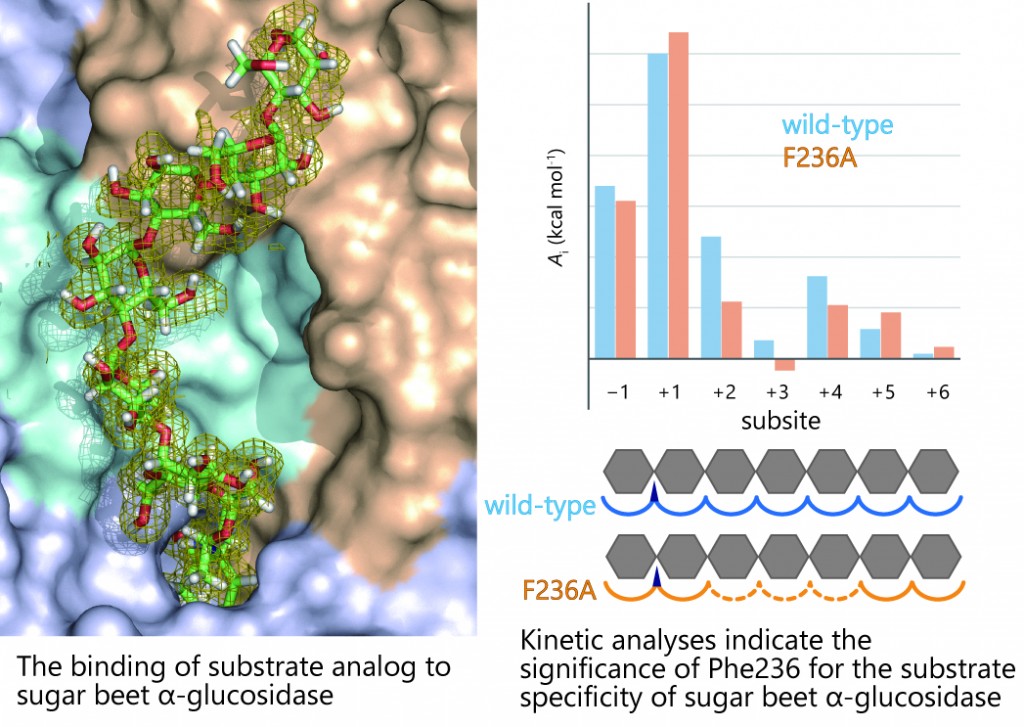
Among enzymes under GH31, our focus is on plant α-glucosidases. The reason of our choice is that plant enzymes can hydrolyze long-chain carbohydrates (e.g. long-chain maltooligosaccharides and starch). As ordinary α-glucosidases generally act on short-chain maltooligosaccharides, plant α-glucosidases are considered to possess a “unique structure factor that recognizes long-chain carbohydrates”. Our three-dimensional structure analyses and protein engineering studies on α-glucosidases derived from rice and sugar beet revealed the structure factor in question. Based on the obtained results, we identified the potential function of α-glucosidases as a key enzyme in starch granule degradation observed in seed germination, which enabled us to suggest a new metabolic mechanism of starch granules.
GH 66
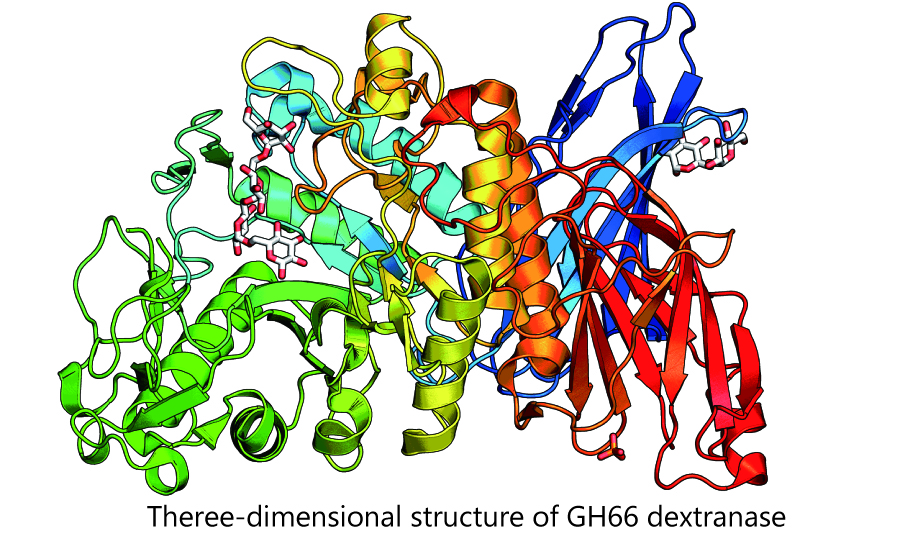
As for GH66 enzymes, we have been examining dextranase, which hydrolyzes dextran. We were the first to succeed in disclosing the three-dimensional structure under the GH66 and also in working out the reaction mechanism. Furthermore, we have proposed the “proenzyme activation phenomenon”, an increased activation triggered by protease-induced degradation, in a dextranase. This is the first case in the realm of carbohydrate-related enzymes to the best of our knowledge. Another noted discovery is a new dextranase that produces cyclic sugar. As cyclic sugar is applicable to various use, we are currently studying their productive mechanism.
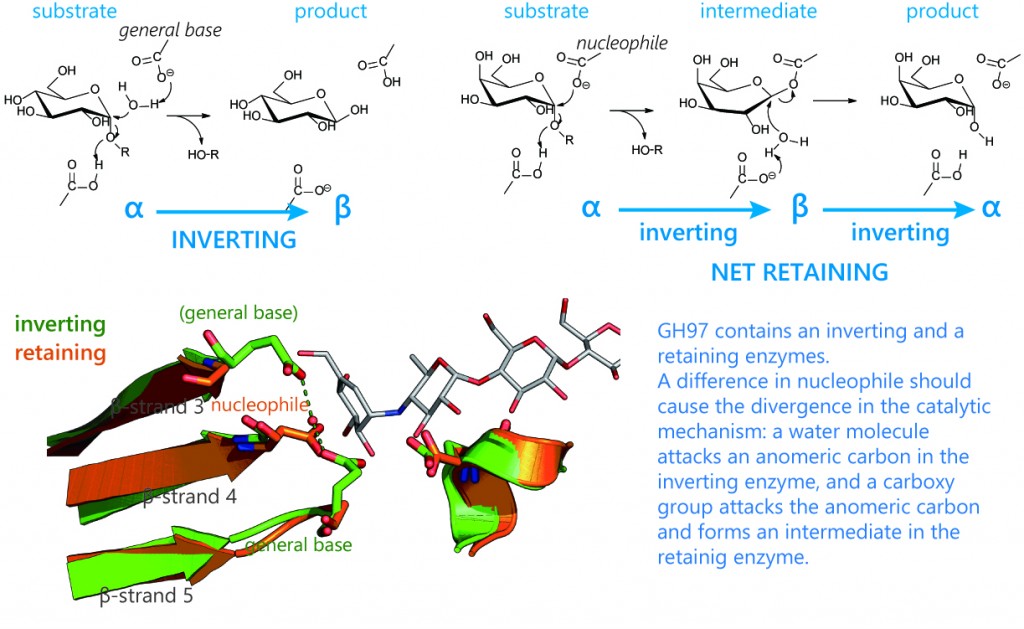
GH97
GH97 is a quite unique family containing a retaining and an inverting enzymes. During the catalysis, the former retains the anomeric configuration, while the latter inverts it. In general, enzymes in a GH family conserve a catalytic mechanism, and GH97 is thus unique from other GH families. At our laboratory, we are working on the catalytic mechanisms of retaining and inverting enzymes while keeping the possibility of molecular evolution within the focus.
Apart from the carbohydrate-related enzymes introduced as above, we are also engaged in researches of carbohydrates. Some examples in this area are enzyme recognition of α-glucosidase inhibitor (a potential diabetic medicine), synthesis of enzyme for oligosaccharide using organic solvents, and discovery of a new metabolic system of 1,5-anhydro-D-fructose produced by algae and other organisms.